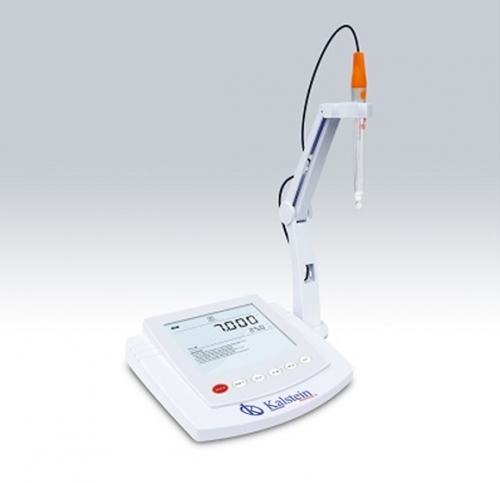
A pH meter is an instrument used to measure the acidity or alkalinity of a solution. The information provided by this equipment expresses the degree of acidity of an acid or base in terms of the activity of hydrogen ions.
The pH is the unit of measurement that describes the degree of acidity or alkalinity and is measured on a scale ranging from 0 to 14. An approximate indication of the pH can be obtained using indicators or pH ribbons, which change color as a function of the variation of the pH level, but these indicators present limitations in terms of accuracy and can be difficult to interpret correctly in dark or colorful samples, and this error can be largely corrected through the use of pH meter.
How is a pHmeter composed?
A pHmeter consists of three essential parts: a pH measuring electrode, a reference electrode and a high input impedance meter. The reference electrode has a known, constant and stable potential.
The pH electrode can be considered as a battery, with a voltage that varies according to the pH of the measured solution. The pH-determining electrode is a hydrogen ion sensitive glass bulb, with a millivolt output that varies according to alterations in the relative concentration of hydrogen ions inside and outside the bulb. The output of the reference electrode does not change with the activity of hydrogen ions. The pH electrode has a very high internal resistance, which makes it difficult to measure voltage variation with pH. Therefore, the impedance of the pH meter input and the dispersion resistances are important factors.
How should a pHmeter be used?
You should note that a pH meter is a very useful device and is currently the most accurate tool to determine the pH of a sample. If you want to make sure you get the most accurate reading possible, you’ll need to follow a number of simple steps, such as preparing the materials needed for measurement, calibrating and testing the equipment.
Preparation for calibration
- Turn on the pH meter
- Clean the electrode with distilled water. Once this clean dries with a lint-free cloth intended for that purpose.
- Prepare the calibration solutions, or use the ones provided by the commercial house.
Calibration of pHmeter
- Place the electrode in the pH 7 gage solution and start the measurement process.
- Once the reading stabilizes, it marks the pH level
- Rinse electrode with distilled water
- Place the electrode in the relevant calibration solution and start the measurement process.
- Redial the pH level.
- Rinse the electrode.
Using the pH meter
- Place the electrode in the sample and begin to measure.
- Mark the pH level.
- Clean the electrode after use.
What do we offer you in Kalstein?
At Kalstein we are MANUFACTURERS of laboratory equipment of the highest quality and with the best technology. In this opportunity we present you pH meter of precision table, calibration of 1 to 5 points, automatic temperature compensation, the menu of configuration contains 9 options, precision: 0,002 pH This device has the following features: HERE
- The precision desktop pH meter is equipped with a large backlit LCD display.
- Automatic recognition 1-5 point calibration for USA, NIST and DIN buffers.
- The automatic diagnosis of the electrode shows the slope and pH compensation.
- Automatic temperature compensation ensures accurate readings across the range.
- The expired calibration alarm prompts the user to calibrate the meter regularly.
For more information we invite you to take a look at: HERE