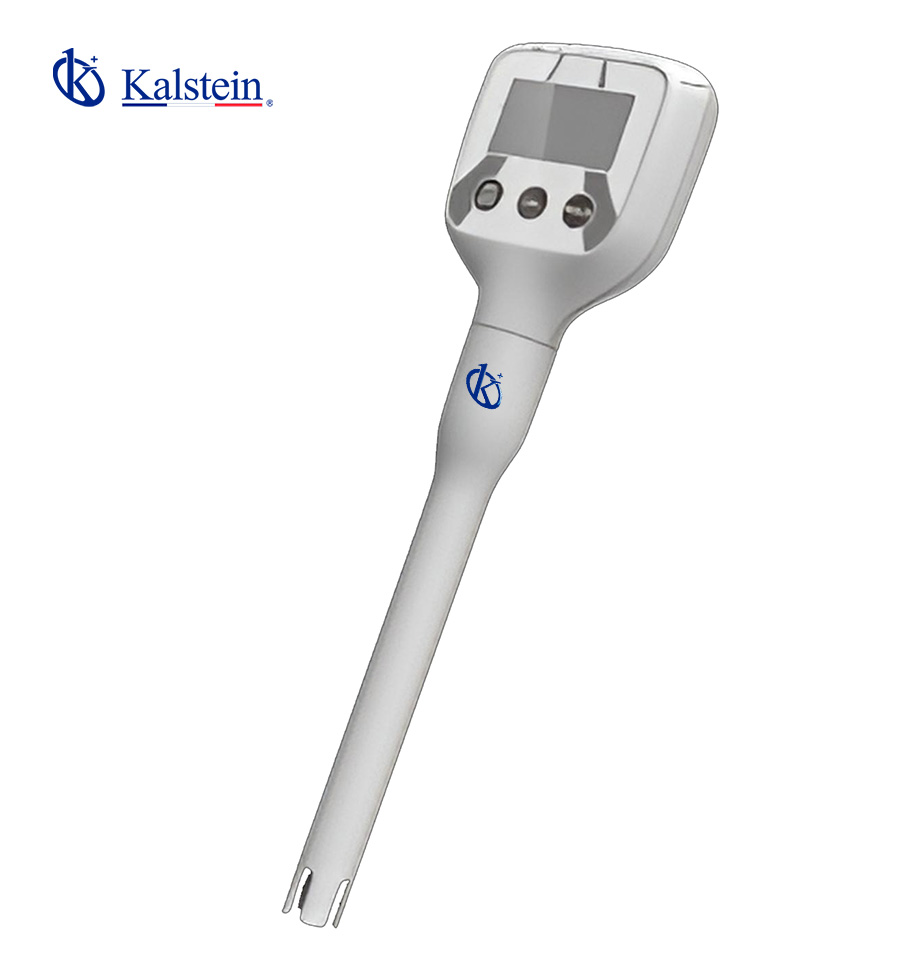
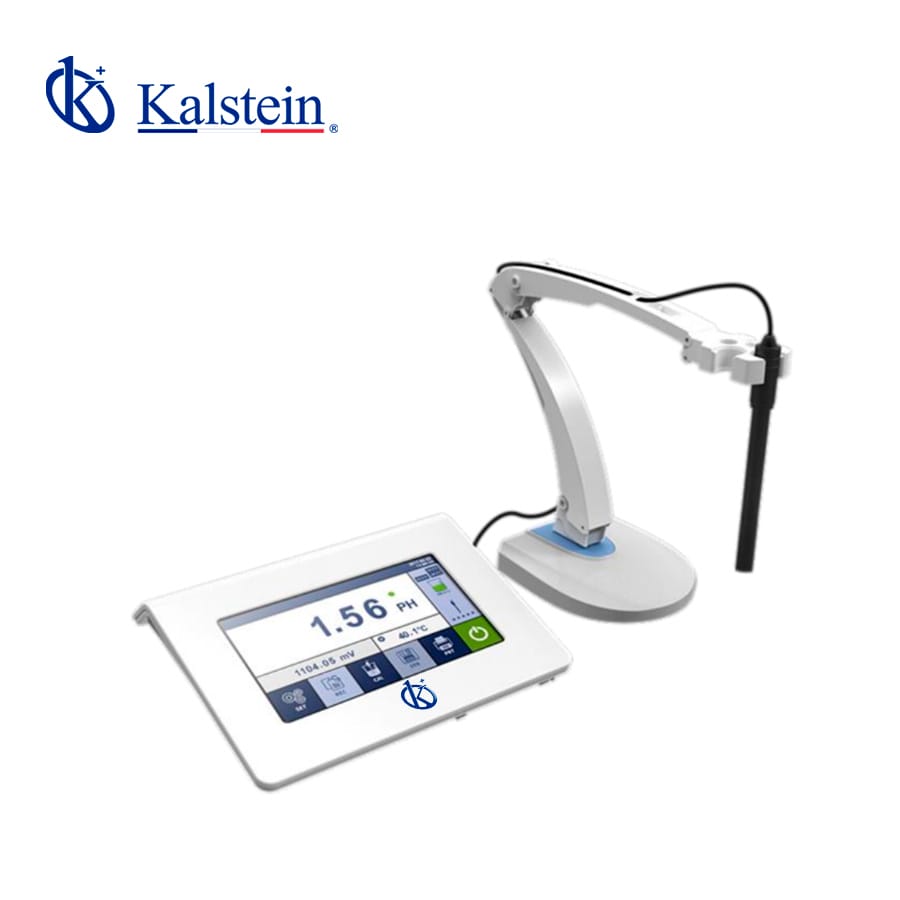
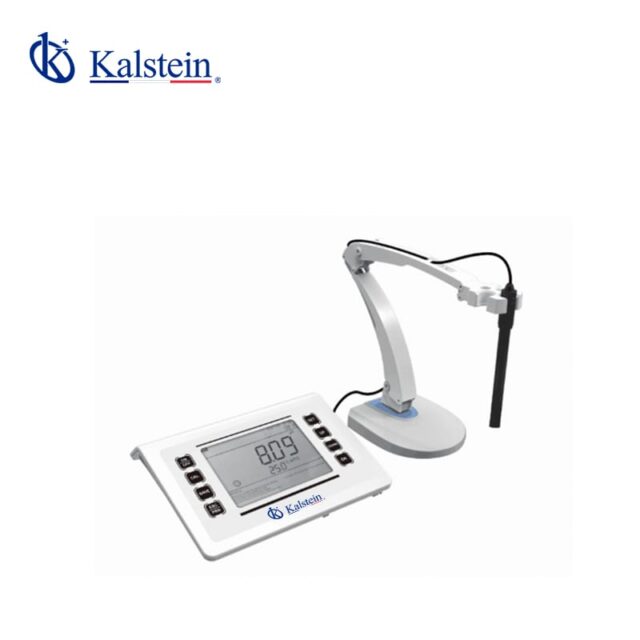
PH meters
Do you need precise and reliable pH measurements for your laboratory work? Look no further than our top-quality pH meters, essential tools in any laboratory setting. Our range of pH meters is designed to provide accurate readings for various samples, ensuring the success of your experiments and analyses. With user-friendly interfaces and durable construction, our pH meters are easy to use and built to last, making them a valuable addition to your lab equipment.
Whether you are working on environmental research, food and beverage analysis, or pharmaceutical testing, having a dependable pH meter is essential. Trust in our selection of pH meters to deliver consistent and precise results, allowing you to carry out your work with confidence and efficiency. Don’t compromise on the quality of your pH measurements – invest in a pH meter from our collection and experience the difference in your lab work.

Types of Ph meters Your Laboratory may need
table pH meters
Within the category of pH meters, there is a subcategory that is essential for achieving accurate and reliable measurements: Sobremesa pH meters. Sobremesa pH meters are designed to be placed on a flat surface, providing stability and precision during pH testing processes. These meters are commonly used in laboratories, research facilities, and industrial settings where consistent and dependable pH measurements are critical.
Sobremesa pH meters offer various features such as easy-to-read displays, automated calibration, and compatibility with different types of electrodes. They are user-friendly and provide quick and accurate results, making them an indispensable tool for professionals working in chemical analysis, environmental monitoring, and quality control. With their compact and durable design, Sobremesa pH meters are ideal for daily use in demanding laboratory environments, ensuring efficiency and reliability in pH testing procedures.
Portable
When it comes to pH measurement on the go, portable pH meters are the ideal solution for laboratories and fieldwork. These compact devices are designed to provide accurate pH readings with convenience and ease of use. Designed for portability, these portable pH meters are lightweight and easy to carry, making them perfect for on-site measurements and quick analysis.
Portable pH meters from Kalstein offer reliable and precise measurements, ensuring accuracy in various applications. With sturdy construction and user-friendly interfaces, these devices are suitable for professionals in the laboratory and field settings. Whether you need to monitor pH levels in water, soil, or food samples, our portable pH meters provide the versatility and performance you need for efficient testing and analysis. With advanced features and reliable technology, our portable pH meters deliver consistent results for your pH measurement requirements.
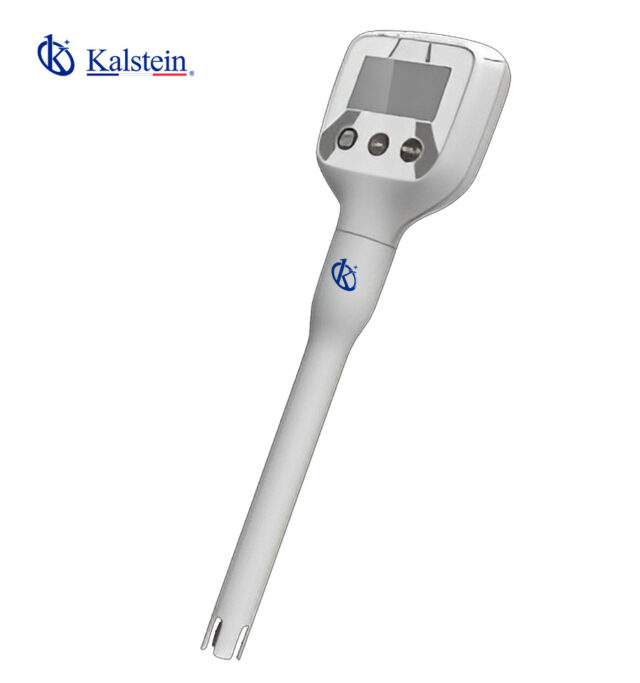
PH METER KALSTEIN
At Kalstein you can find the ideal Ph meters for Your Laboratory
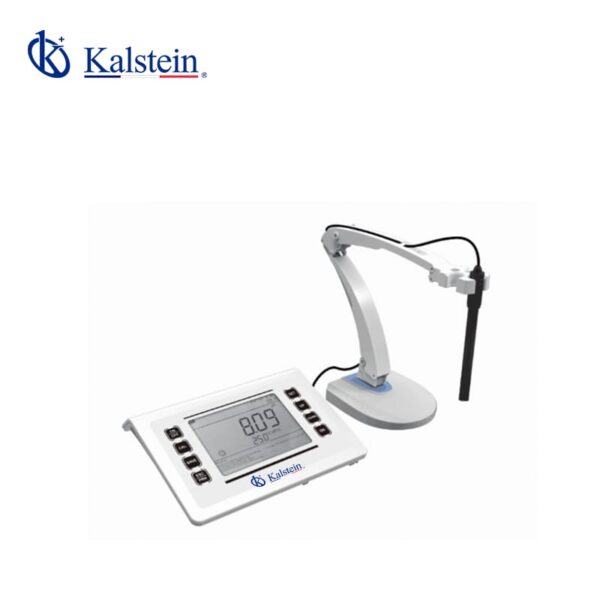
PH Meter YR01821
This advanced measurement instrument features a 6.5-inch LED screen with a concise and user-friendly interface. It includes a built-in microprocessor chip offering intelligent functions such ...
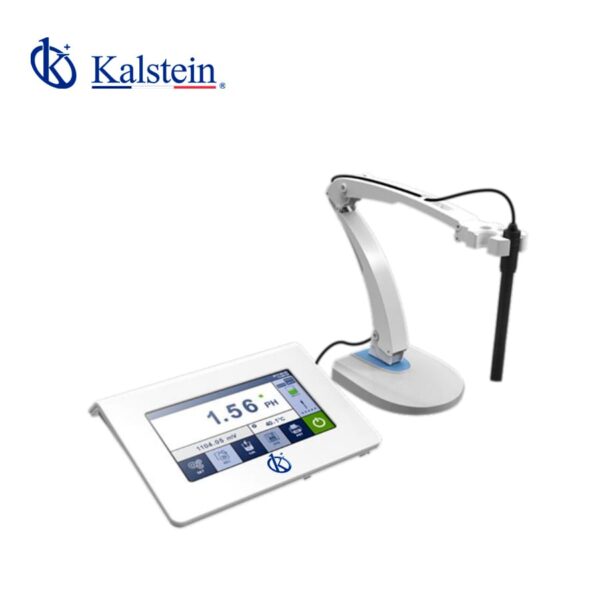
YR01825 Benchtop pH / Ion Meter
This advanced measurement device features a 7-inch colored capacitive touch screen with a high resolution of 1024×600 and excellent sensitivity, providing a superior user...

YR01822 Benchtop pH Meter
This sophisticated measurement device boasts a 6.5-inch LED screen with an intuitive and easy-to-use interface. It features a built-in microprocessor chip that provides smart functions such as automat...
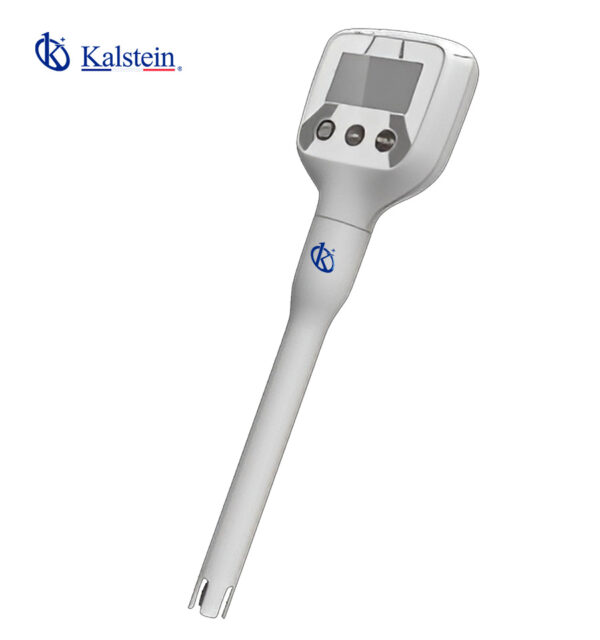
Pocket pH Meter – ° C / ° F YR01800 – YR01800-1
Series-Pen Type- pH Meter YR01800 is Compact mini design, making it highly portable and convenient for on-the-go use. It incorporates intelligent functio...
Our PH meter best seller
This sophisticated measurement device boasts a 5-inch LCD screen with a 150° viewing angle, ensuring clear readability from up to 5 meters away. It features a built-in microprocessor chip that provides intelligent functions such as automatic calibration, Automatic Temperature Compensation (ATC), data storage, clock display, USB output, function settings, automatic power-off, and a low voltage alarm.
Equipped with two USB interfaces, the device supports PC connections, battery charging, and data transfer. The 3.5mm electrode interface simplifies electrode connection. The device can automatically recognize different electrodes, and those with built-in chips can store and retrieve calibration data upon connection.
It supports the automatic recognition of 15 buffers with options for Europe & USA, NIST, and China, and allows for 1, 2, or 3-point calibration. The device can store up to 200 sets of data, including readings, GLP data, date, and time. Its rechargeable battery provides up to 30 hours of use, and the device will automatically power off after 10 minutes of inactivity.
| Model | YR01826 | |
|
ph
|
Measuring range | (-2~19.999)pH |
| Resolution | 0.1/0.01pH | |
| Accuracy | ±0.02 pH | |
| Input current | ≤2×10^-12 A | |
| Input impendance | 21×10^-12 Ω | |
| Stability | ±0.002 pH/3h | |
| Temp. compensation range |
(0~100) °C, auto/manual
|
|
|
mV
|
Measuring range |
-1999.9mV~0~1999.9mV
|
| Resolution | 0.1mV | |
| Accuracy | ±0.03% FS | |
|
Temperature
|
Measuring range | (-10~110) C |
| Resolution | 0.1°C | |
| Accuracy |
5~ 60°C: range: ±0.4°C; other range: ±0.8℃
|
|
| Calibration | 1, 2, 3 point | |
|
Other Technical Parameters
|
Display |
5-Inch white backlit LCD screen
|
| Data storage | 200 sets | |
| Power | DC 5V/1A | |
| Communication connector |
Micro USB & Standard USB
|
|
| Dimension/N.W. |
180 x 130 x 14mm/280g
|
|
|
Working Condition
|
Environment Temp. | 535 °C |
| Environmental | ≤85% | |
| IP Grade | IP54 | |
|
Meter Kits
|
1) pH meter | |
| 2) Electrode holder | ||
| 3) Two-in-one electrode that consists of pH combination electrode and temperature probe. | ||
| 4) pH buffer solution | ||
| 5) Power adapter | ||

Analysis of the best Ph meters for Your Laboratory

How does a pH meter work?
A pH meter or pH meter is an instrument used to measure the acidity or alkalinity of a solution. The information provided by this equipment expresses the degree of acidity of an acid or a base in terms of the activity ...

Functions of pH Ion Meters in the Cosmetic Industry
In the manufacture of cosmetic products there are three essential aspects that must be addressed, the first, the elaboration of products pleasant to the touch and to t...

PH meter or pH meter: How should it be used?
A pH meter is an instrument used to measure the acidity or alkalinity of a solution. The information provided by this equipment expresses the degree of acidity of an acid ...

PH meter,and human body pH
PH is a parameter used to measure the concentration of the hydrogen ion [H +] in a solution. In definition pH, it is the negative logarithm of base 10 of the activity of the [H +]. PH values vary on a sca...
KALSTEIN UPDATED
Guidelines for you to become an expert in Ph meters
The Ph meters equipment are essential products in Your Laboratory, we provide you with guidance and recommendations for a better use, so you can work like an expert.
Importance of pHmeter in a laboratory
pHmeter: Technology and accuracy
pH meter vs. dissolved oxygen meter
What are the differences between: PH Meter, Ion Meter and Conductivity Meter?

Frequently asked questions from our customers about Ph meters
The delivery time of your Kalstein product will depend on the following:
- Whether the equipment you are interested in is in stock or if it needs to be manufactured.
- The type of freight you have chosen, which can be either air or sea.
- Equipment in stock:
– Delivery Time (Air): 15-30 days.
– Delivery Time (Sea): 45-60 days.
- Equipment not in stock:
– Delivery Time (Air): 30-60 days.
– Delivery Time (Sea): 60-90 days.
You can make your purchase through:
- By email: [email protected]
- By phone: +33 (0) 1 70 39 26 50
- Online shopping: Through the official Kalstein website in your country.
At Kalstein, we provide our customers with inductions and technical support through new online methods. You can visit our induction videos, technical assistance, and guidance provided by a Kalstein team through our Youtube channel (Kalstein English). HERE
Send us a direct message and one of our agents will contact you
Ph meters
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Sed dignissim placerat mauris cursus laoreet. Nam feugiat lacus ex, at fermentum sapien accumsan nec. Curabitur auctor porttitor mi non malesuada. Aenean condimentum, purus vitae rhoncus imperdiet, justo eros aliquam ipsum, at egestas leo diam eget libero.

Catalog of models of Ph meters on offer.
-

Electric Heating Drying Oven YR06446
-

Electric Heating Drying Oven YR05259-2
-

Electric heating drying oven YR05248 // YR05255
This product has multiple variants. The options may be chosen on the product page -

Electric heating drying oven YR05244 // YR05247
This product has multiple variants. The options may be chosen on the product page -

Mini Centrifuge With Large Capacity YR012G
-

Tabletop High Speed Centrifuge YR0137-2 – YR0137-3
This product has multiple variants. The options may be chosen on the product page -

Gel Card Centrifuge YR142-3 – YR142-3-1
This product has multiple variants. The options may be chosen on the product page -

Tabletop High Speed Centrifuge YR019-TG
This product has multiple variants. The options may be chosen on the product page -

Intelligent Electric Wheelchair YR06432
-

Electric Wheelchair YR05445
-

Electric Wheelchair YR05443
-

Electric Wheelchair YR05442
-
Electric Wheelchair YR05444
-

Electric Wheelchair YR05441
-

Electric Wheelchair YR05440
-

Electric Wheelchair YR05439
Descubre más de nuestro catálogo
Tipos de Ph meters

[Producto] A
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.

[Producto] B
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
Find out more about Ph meters with our guides.
Ultrasound Scanner: Real-Time Imaging Diagnosis for Reliable Results
The ultrasound scanner is a fundamental tool in the field of medical diagnostics. This device not only allows for the...
Dental Units: Complete Stations for Modern Dental Care
Dental units have revolutionized how dentists perform their procedures. Over the years, technological advancements have transformed these stations into key...
Transilluminator: Essential Technology for DNA and RNA Detection in Laboratories
The analysis of nucleic acids, such as DNA and RNA, is a critical task in any molecular biology laboratory. In...